Issue 009, April 13, 2021
Megan K. Puglia, Ph.D., Senior Research Chemist
X-ray Fluorescence Spectroscopy (XRF) is a powerful tool used to determine the elemental composition of samples across many industries including geology, environmental management, the polymer industries, and metallurgy. In the metals and precious metals industry, XRF is often used as a quality control measure to confirm the elemental composition of raw materials used in final parts. Depending on the instrument and sample matrix, XRF is capable of detecting most elements greater than 1 ppm. However, limits to perform quantification are greater and can range from 100 to 1000ppm (0.01 to 0.1 wt.%).[1] XRF is used at Deringer-Ney Inc. in conjunction with inductively coupled plasma optical emission spectroscopy (ICP-OES) to quantify the composition of materials fully.
XRF machines bombard samples with X-ray radiation absorbed by electrons in the atoms’ inner orbitals. Absorbing this X-ray energy causes these inner electrons to be ejected from the atom leaving behind a vacancy in the inner electron shell. An electron then fills this vacancy from a higher energy level, which causes a release of energy in the form of a photon. This detectable photon has an energy equal to the difference in energies between the initial and final orbital of the electron, making the energy value distinctive to a specific element. The electron that filled the vacancy of the initially ejected electron leaves behind its own vacancy that is similarly filled by an electron from a higher energy level, again releasing fluorescent X-rays, and so on (Figure 1).[2]
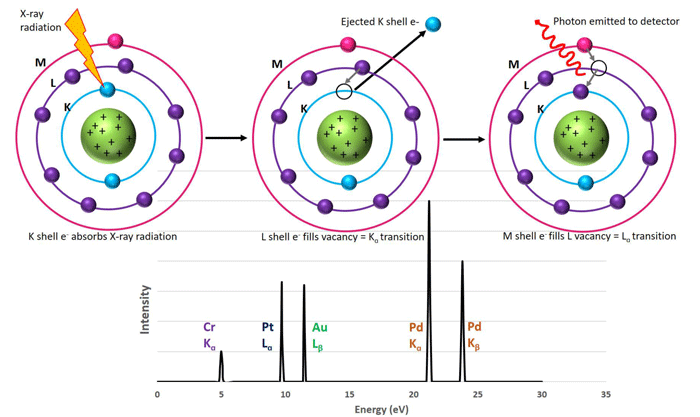
Figure 1. Scheme depicting K, L, and M electron energy levels of an atom. Left to right: An initial x-ray radiation of an inner K electron causing ejection and a vacancy in the inner energy level. An L electron drops down to fill this vacancy and in doing so releases a photon of a specific energy (known as a Kα transition). The L vacancy is then filled by an M electron (Lα transition) and another photon is released to the detector with a different energy than the first. Both photon energies are specific to the element being ionized and their detection leads to a plot similar to the bottom panel.
As the cascade of electron vacancy replacements is completed, the sample emits a number of different, characteristic fluorescent photons which are detected by the XRF machine. This is accomplished by measuring either the energy or wavelength of the photons. The latter technique provides higher resolution measurement and is preferred for quantitative analysis. The XRF is then able to produce a spectrum of the total intensity of photons collected at each specific wavelength that is unique for each element. Elements present in a sample are identified by peak position and quantified by comparing the peak’s intensity to a known standard.
Some limitations to XRF that should be considered are relatively slower sample analysis times (up to 30 minutes per sample), as well as higher limits of detection in comparison to ICP-OES, especially for lighter elements. The XRF depth of analysis into a sample increases with increasing elemental X-ray energy, and therefore it is essential to maintain homogenous samples. Strictly uniform sample preparation procedures must be followed as differences in density, grain size, and surface roughness can also influence results. A smooth sample surface is often achieved by grinding and polishing the sample. Like ICP-OES, it is important to compare unknown samples to matrix-matched standards to mitigate matrix affects in the analysis. In the case of alloys produced by Deringer-Ney Inc., a collection of alloy-specific standards has been created and carefully curated for this purpose.
XRF has many advantages for metallurgical analytical labs that include simple sample preparation that does not generate chemical waste, as well as relatively low up-keep cost. XRF is nondestructive so samples can be analyzed via XRF and then subsequently analyzed by other methods if desired. XRF is able to analyze solid samples, powders, pellets as well as liquid samples. Recent advancements in XRF technology have led to hand-held XRF guns for in-situ analysis of unknowns as well as high-throughput stationary machines that are excellent for quality assurance.[3]
References:
[1] Kadachi, A.N. and Al-Eshaikh, M.A. (2012), Limits of detection in XRF spectroscopy. X-Ray Spectrom., 41: 350-354. https://doi.org/10.1002/xrs.2412
[2] Guthrie, James M, and Jeffrey R Ferguson. “Overview of XRF.” The Archaeometry Laboratory at the University of Missouri Research Reactor, University of Missouri Research Reactor, Aug. 2012, https://archaeometry.missouri.edu/xrf_overview.html.
[3] Temitope D. Timothy Oyedotun (2018) X-ray fluorescence (XRF) in the investigation of the composition of earth materials: a review and an overview, Geology, Ecology, and Landscapes, 2:2, 148-154, DOI: http://dx.doi.org/10.1080/24749508.2018.1452459
