Issue 017, September 20, 2021
Megan K. Puglia, Ph.D., Sr. Research Chemist
Materials engineered specifically to cooperate with biological systems for a multitude of purposes are often called biomaterials. Biomaterials include metals, metal alloys, polymers, ceramics, bio-glasses, and everything in between. The biomaterials field of materials science continues to grow rapidly as it touches on life-changing medical applications including: implant materials, therapeutics, tissue scaffolds, sensing devices, and diagnostic probes.
In this field, the term bioinert describes a material that does not react or initiate a host reaction when in contact with biological tissue or systems. This usually means that the material is able to make direct or close contact with host tissue. Titanium and its alloys, stainless steels, and most noble metals are often described as bioinert. The contrasting adjective, bioactive, designates materials that initiate a host response or a material-tissue interfacial reaction. Bioactive materials often instigate chemical bonds between host tissue and themselves through different biophysical and biochemical reactions. Biotolerant materials are separated from the host tissue by a fibrous capsule generated by corrosion products or chemicals released between the implanted material and host tissue. These materials are not rejected by the host tissue but they are separated from direct contact by this fibrous tissue layer.[1]
Biodegradable materials are able to dissolve, on various time-scales, in bodily fluids and tissues. These materials have applications in controlled drug release, sutures, and wound healing.[1] Confusingly, each of these terms is sometimes used interchangeably with the word biocompatible, broadly meaning the material is not harmful to the host-tissue.
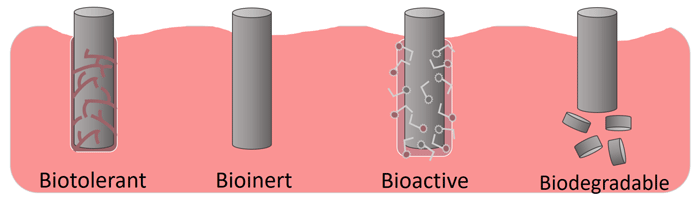
Figure 1: Simplified scheme depicting different reactions between materials and host tissue depending on the specific system under consideration.
It is important to note that these terms are not universal, but application and system dependent. If a material is bioinert as a dental implant, for example, it is not necessarily bioinert as a stimulation electrode, and vice versa. Likewise, a material may be bioinert in one type of host-tissue, but bioactive in another.
Prof. David F. Williams, former editor in chief of Biomaterials, argues that it is important for authors and researchers to define biocompatibility in terms of a specific, time-dependent material-host system that has been studied, and not a material property per se.[2]
It is important to keep this distinction in mind when viewing the Figure 1 schematic, as well as when designing medical materials. Many different factors can affect a material’s status as bioinert in the same, or similar, host systems. These include impurities, surface roughness, corrosion resistance, surface passivation, and different timescales. With varying host systems, factors such as surface energy, and cytotoxicity can also affect biocompatibility, in addition to the aforementioned factors.
Noble metals and metal alloys offer excellent mechanical properties, toughness, X-ray radiopacity, mechanical stability, and formability for medical implants. Base metals including titanium and its alloys, austenitic stainless steels, and cobalt-chromium alloys have been used successfully as implant materials for decades.[3] Noble metals like platinum, palladium, gold, and iridium are all relevant to medical device application due to their low reactivity.[4]
Deringer-Ney Inc. (DNI) offers a wide variety of noble metals and alloys for various medical applications. Notably, DNI has developed the Paliney® In Vivo family of alloys engineered specifically for implantable and interventional medical devices. These alloys exhibit similar mechanical and radiopaque characteristics as the platinum and gold alloys they replaced. Paliney 500 and 1100 have both been studied with specific International Organization for Standardization (ISO) 10993 tests to better understand their cytotoxicity, irritation, sensitization, and systemic toxicity in controlled, comparable systems. The ISO 10993 tests, as shown in Table 1, are carefully chosen, well respected test methods, however it is important to understand that their results cannot universally predict all material-system interactions.
Paliney In Vivo materials have been used successfully in microcoil catheter tips and radiopaque marking devices for interventional medical devices. Other DNI alloys have been used in implantable pulse generators, blood sensors, imaging systems, hearing aids, and intravascular surgical devices. DNI continues to work with customers to design and study new materials for medical applications by carefully focusing on the specific material-host system under consideration.
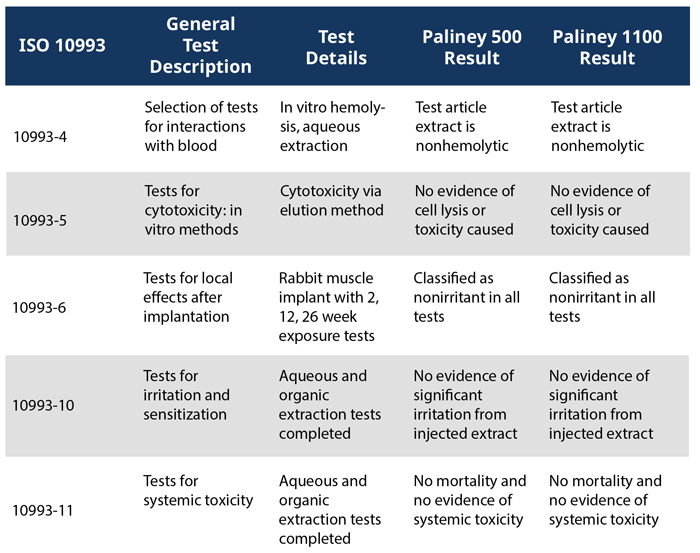
Table 1. Summary of specific ISO 10993 tests performed in order to better understand Paliney In Vivo alloy performance in different systems. For additional material details see reference 5, and for additional testing details refer to ISO 10993.
References:
[1] Ødegaard, K. S., Jan T., and Christer W. E. “Structural and Biomedical Properties of Common Additively Manufactured Biomaterials: A Concise Review.” Metals, 2020, 10, (12), 1677. DOI:10.3390/met10121677.
[2] Williams, D. F. “There Is No Such Thing as a Biocompatible Material.” Biomaterials, 2014, 35 (38), 10009–10014. DOI:10.1016/j.biomaterials.2014.08.035.
[3] Barfeie, A., Wilson, J., Rees, J. “Implant Surface Characteristics and Their Effect on Osseointegration.” British Dental Journal, 2015, 218 (5). DOI:10.1038/sj.bdj.2015.171.
[4] Puglia, M. K. “What Determines Deringer-Ney Alloy Nobility?” Deringer-Ney Tech Briefs, December 16, 2020, https://www.deringerney.com/resource-library/.
[5] https://www.deringerney.com/assets/1/7/data-sheet_Paliney_500__alloy_Invivo_Medical_wire.pdf
https://www.deringerney.com/assets/1/7/data-sheet_Paliney_1100_alloy_Invivo_Medical_wire.pdf
