Issue 003
December 16, 2020
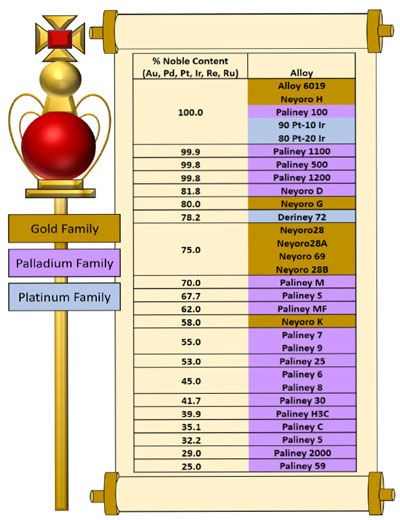
Deringer-Ney Inc. (DNI) invents and produces state-of-the-art precious metal alloys that are used in sensors, electronics, medical devices, automobiles, and semiconductor test equipment. Most of the DNI alloys contain noble metals to ensure resistance to chemical attack, low electrical contact resistance, and longevity of the parts formed from these alloys. DNI considers gold, platinum, palladium, rhenium, ruthenium, and iridium to be noble metals, and assigns alloy nobility based on the total weight percentage of these six elements in the alloy (Figure 1). Other transition metals not considered noble metals are usually considered to be base metals. DNI works carefully with these noble metals to design stable alloys that are specifically suited for the many different industries they serve.
What determines a noble metal? In general, a lack of chemical reactivity, is what sets the noble metals apart from their base metal counterparts. Noble metals do not readily form bulky oxides in the presence of oxygen. This quality makes noble metals an ideal constituent of jewelry, coins, and sensors. Most noble metals are good electrical conductors that also resist surface reactions, and are therefore used in electrical contacts to ensure low contact resistance over their lifespan. Despite being relatively inert, many noble metals have been found to be good chemical reaction catalysts. Platinum is used in catalytic converters to oxidize carbon monoxide, hydrocarbons, and nitrogen oxides in internal combustion engines; palladium is used as a catalyst for hydrogenation / dehydrogenation reactions.[1] The catalytic nature of certain noble metals can create challenges in sliding contacts due to their tendency to create friction polymers from adsorbed hydrocarbons.
Rankings of noble metals can vary from source to source using different theories to justify nobility such as d-band properties, ionization potentials, bulk metal surface re-activity, oxophilicity, and lack of reactivity to single strong acids. Most lists include rhodium and osmium due to their high electronegativity values and associated resistance to oxidation. Some lists may even include silver, copper, and mercury due to their fully filled d-orbitals.[2] While not considered noble metals by DNI, many of the alloys listed in Figure 1 also contain base elements like silver and copper and still maintain low reactivity.
Another determinant of metal nobility is the galvanic series. The galvanic series ranks metals based on their standard reduction potentials and their ability to resist corrosion within a galvanic cell. For such an experiment, two different metal electrodes are placed into an aqueous electrolyte and are connected together by a conductor. Noble metals have large positive reduction potentials (> +0.4V), and therefore will act as the cathode. In comparison, base metals (< +0.4V) will release electrons, functioning as the anode, and experience surface corrosion (galvanic corrosion).[2]
Some argue that the most distinct feature of noble metals is their high electronegativity values which are derivative of their high effective nuclear charge and, in some cases, partially filled s-orbitals. These high electronegativity values lead to weakly-polar covalent bonds formed between noble metals and oxygen, resulting in their resistance to oxidation. Gold maintains the highest electronegativity value of all metals, making it least likely to bond with electronegative elements like oxygen.[2]
Not all applications require the same magnitude of nobility. Contacts made for marine applications, of course, require a higher nobility content than contacts made for climate-controlled environments. For this reason, DNI maintains an alloy portfolio that covers a broad spectrum of alloy nobility, and carefully matches the nobility of the alloy to the application. Understanding the unique and superior properties of different noble met-als has allowed DNI to manipulate them into advanced noble alloys for advanced applications.
References
[1] Muroi, Takashiro. Role of Precious Metal Catalysts. INTECH Open Access Publisher, 2012.
[2] K.P. Kepp, Chemical Causes of Metal Nobleness. ChemPhysChem 2020, 21, 360
