Issue 024, January 03, 2022
Megan K. Puglia, PhD, Sr. Research Chemist
Implantable electrodes encompass a variety of devices that impart an electrical signal to body tissue, or record nerve signals sent by the body, with some newly marketed bioelectrodes even capable of both roles. Since the introduction of the cardiac pacemaker, implantable electrodes have become acceptable medical treatments for epilepsy, Parkinson’s disease, diabetes, arthritis, paralysis, and pain modulation, amongst many others.[1] Research continues to improve upon these available treatments while developing new implantable electrodes with objectives like vision repair, diabetes modulation, and rheumatoid arthritis relief.
The success of an implantable electrode is strongly influenced by the choice of electrode material, as there are a number of material characteristics to consider: electrical and thermal conductivity, biocompatibility, stability, radiopacity, and machinability. Precious and noble metal alloys exhibit a number of these imperative characteristics making them long-time favorites for successful implantable electrode design.
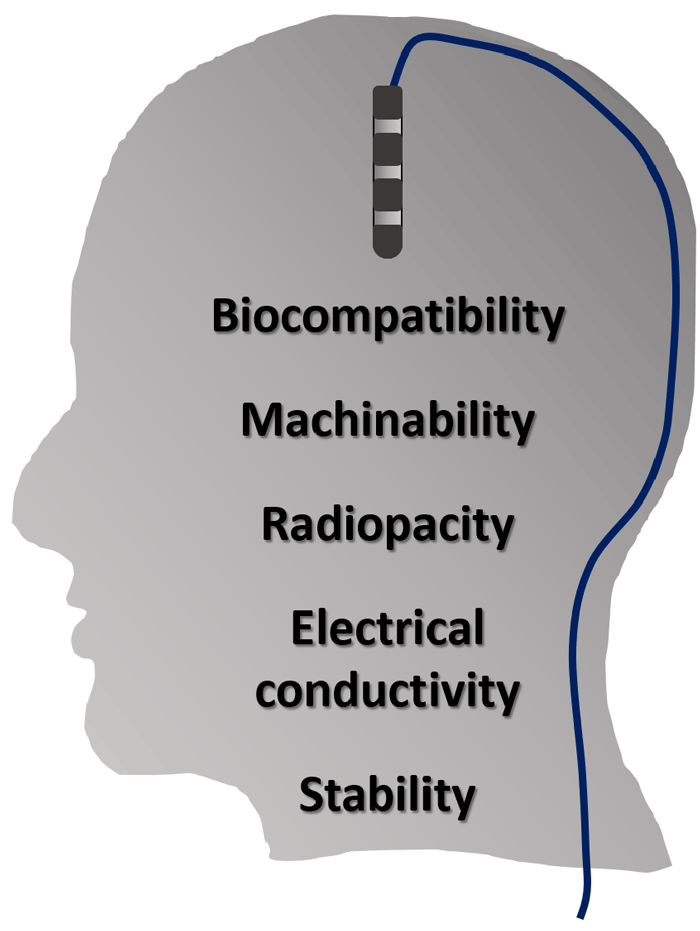
Figure 1: Schematic of a DBS electrode and important electrode material characteristics.
Metal alloys like platinum and platinum-iridium are often chosen for implantable electrodes due to their electrical conductivity and charge injection capabilities. High charge injection capacities (CIC) allow for smaller electrodes that can still inject appropriate charges without causing adverse chemical reactions. Optimal electrode materials have high CIC values and low impedance values, together enabling the continual miniaturization of implantable electrodes. Low electrode impedance values also reduce background noise in signal recording for systems like intracortical recording devices.
The electrode material must also be chosen for stability and biocompatibility in its intended biological milieu. In general, precious metals show excellent compatibility and stability in their intended systems, due to their resistance to corrosion and oxidation. Noble metals like gold, platinum, and iridium generally do not require any sort of surface passivation or functionalization prior to implantation into the human body. Gold and platinum alloys are also unlikely to degrade under applied current. Platinum alloys have a history of success in medical implants and electrodes, largely for this reason.[2]
For specific implantable electrodes it is important that the material maintain a certain level of dimensional stability and mechanical behavior, requiring strength and predictable load-deflection characteristics. This is often achieved by using platinum-iridium alloys[3], which are stronger and harder than their pure platinum counterparts, but maintain the desirable attributes already discussed.
Precious metal alloys also offer radiopacity, an important characteristic for implantable electrode materials. After implantation, it is common to view implanted electrodes radiographically over time in order to monitor their position and any tissue responses.[4] Platinum and gold alloys both offer high X-ray radiopacity.
Precious metals alloys are used today in a very wide variety of implantable medical electrodes, and some clinically available devices and their applications are listed in Table 1. Platinum and platinum-iridium are the most commonly used precious metal implantable electrode components and can be found in pace makers, nueromodulators, and deep-brain stimulation (DBS) electrodes. Gold wires have been used in neurotrophic electrodes[5] and cochlear implants.
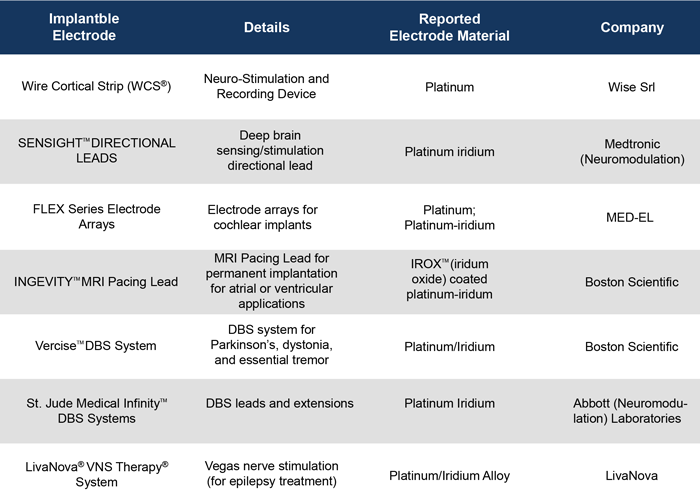
Table 1: Clinically available implantable electrode systems made using various precious metal alloys.
Precious metal coatings like iridium oxide have become popular in the field of implantable electrodes because of the increased electrochemical surface area they offer, which decreases overall electrode impedance. Lower electrode impedance values can improve device battery life.[6] New research has evaluated precious metal coatings on non-metallic substrates for implantable electrode applications. These coatings claim high surface area, beneficial electrocatalytic effects, and increased compatibility with specific biological tissues and systems[7], but have attendant risks and failure modes common to all coated biomaterials.
DNI is practiced in constructing stable, implantable electrode devices from our precious metal alloys, and can assist with materials selection to design successful implantable electrodes in new devices.
References:
[1] Jalili, R., et al. Implantable Electrodes. Current Opinion in Electrochemistry, 2017 July, 3;1:68-74
[2] Bain, L.J. Materials for Implantable Electrodes. MRS Bulletin, July/August 1986, 24
[3] Brunton E. K., et al. In vivo comparison of the charge densities required to evoke motor responses using novel annular penetrating microelectrodes. Frontiers in Neuroscience, 2015 May, 9:265
[4] Gedded, L. A. & Roeder, R. Criteria for the selection of materials for implanted electrodes. Annals of Biomedical Engineering, 2003, 31: 879-890
[5] Bartels J., et al. Neurotrophic electrode: method of assembly and implantation into human motor speech cortex. J Neurosci Methods. 2008;174(2):168-176.
[6] Sarica, C. , et al. Implantable Pulse Generators for Deep Brain Stimulation: Challenges, Complications, and Strategies for Practicality and Longevity. Frontiers in Human Neuroscience, 2021, 15: 489
[7] Kim, H. S., et al. Noble metal sensitized invasive porous bioelectrodes: advanced medical device for enhanced neuronal activity and chronic alcohol treatment. RSC Adv., 2020, 10, 43514
