Issue 035, May 22, 2022
Megan K. Puglia, Ph.D., Sr. Research Chemist
Base metals and their alloys have long been known to exhibit an ion release phenomenon in specific environments where surface degradation and corrosion causes metal cations to be liberated into the surrounding solution. This degradation mechanism is a critical design consideration for the engineering of biomedical implants, where it can lead to undesirable interactions with surrounding tissues. Additionally, it is relevant for food processing equipment, potable water infrastructure, skin-contacting products, and marine applications. Corrosion resulting in metal ion release occurs differently for different metal alloys and is heavily influenced by the environment as well as corrosion resistance of the metal.
The release of metal ions is associated with other degradation products such as metal hydroxides, phosphates, carbonates, and oxides.¹ Mechanisms of metal ion release include electrochemical corrosion and oxidation, dissolution of surface oxides, and physical processes such as mechanical wear that remove particles into solution.² These corrosion reactions can occur at distinct phases on grain boundaries, between grains with potential differences, the surface passivation layer, and when metal couples exhibiting a galvanic potential are made.
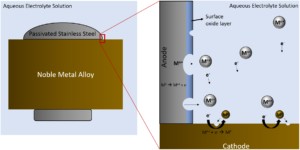
Figure 1: Illustration of galvanic coupling corrosion between a stainless steel screw and noble metal alloy plate. In this scenario the stainless steel has a lower (more negative) potential than the noble metal alloy and therefore becomes the anode resulting in accelerated corrosion. The noble metal alloy cathode will corrode more slowly in this coupling than if it was alone.
Non-passivating base metals are more likely to corrode and release metal ions than passivated base metals, which are more susceptible than noble metals, under similar conditions. Commonly used alloys in the making of prosthetic implants are from the family of passivating base metal alloys, and include: stainless steel based alloys, titanium alloys, and cobalt alloys.¹ Different materials are chosen for different types of implants due to a combination of factors such as strength, passivity, hardness, and resistance to wear.
Stainless steel (SS) is a passivating iron alloy with a minimum of 11% chromium content by weight used in many types of biomedical devices and implants. Chromium oxides form at the surface of passivated SS creating a protective barrier film preventing corrosion. This surface oxide film’s thickness and tenacity is influenced by the type of SS (i.e. austenitic or ferritic), the method of passivation, and the environment. Pitting corrosion, and subsequent metal release, can occur on passivated SS surfaces, especially in the presence of chloride ions that are often found in biological environments.²
Galvanic corrosion of ferritic stainless steels used in dental magnetic attachments is known to occur when they come in close contact with a noble alloy used as the dental root cap. The galvanic corrosion is initiated by the difference in corrosion potential between the two alloys with saliva acting as the corrosive aqueous electrolyte. The noble alloy becomes the cathode, leaving the SS as the anode and resulting in corrosion and subsequent metal ion release,³ illustrated in Figure 1. In the figure, the system is subjected to a conductive biological media causing the SS to act as an anode, releasing metal ions and electrons into solution. Other mechanisms of degradation that disrupt the surface oxide film such as fretting and wear can accelerate this process. The noble metal alloy would act as the cathode in this situation.
Titanium and titanium alloys, like Nitinol and Ti-6Al-4V, have good biocompatibility and are used in medical implants like cranial plates, dental implants, and joint replacement prostheses. Ti-6Al-4V is susceptible to fretting corrosion when used in taper connections of joint replacements, leading to alloy particle release in nearby tissues.⁴
Cobalt-chromium-based alloys containing additions of molybdenum (Mo) or tungsten (W) are used in medical implants like heart valves, spinal rods, and joint replacement prostheses due to their resistance to wear and corrosion. Corrosive wear at the load-bearing implant surfaces for these alloys has been known to release cobalt into the surrounding tissue. Galvanic coupling corrosion can also occur between differently processed Co-Cr-Mo alloys, such as cast versus wrought Co-Cr-Mo.⁴
The release of metal cations due to corrosion and wear particles from biological implants can result in eventual implant failure, pain, and chronic inflammation of some tissues, making it imperative to understand the mechanisms behind these events during device design. Deringer-Ney Inc. has years of experience making biocompatible noble metal alloys as well as machined titanium alloy and stainless steel parts, and can help advise customers on the best materials for their systems.
References:
- Sansone, V. “The Effects on Bone Cells of Metal Ions Released from Orthopaedic Implants. A Review.” CLINICAL CASES IN MINERAL AND BONE METABOLISM, 2013. https://doi.org/10.11138/ccmbm/2013.10.1.034.
- Hedberg, Yolanda S., and Inger Odnevall Wallinder. “Metal Release from Stainless Steel in Biological Environments: A Review.” Biointerphases 11, no. 1 (March 2016): 018901. https://doi.org/10.1116/1.4934628.
- Takahashi, Noriko, Yukyo Takada, and Osamu Okuno. “Galvanic Corrosion between Dental Precious Alloys and Magnetic Stainless Steels Used for Dental Magnetic Attachments.” Dental Materials Journal 27, no. 2 (2008): 237–42. https://doi.org/10.4012/dmj.27.237.
- Hansen, Douglas C. “Metal Corrosion in the Human Body: The Ultimate Bio-Corrosion Scenario.” The Electrochemical Society Interface 17, no. 2 (June 1, 2008): 31–34. https://doi.org/10.1149/2.F04082IF.
