Issue 043, April 25, 2022
Patrick K. Bowen, Ph.D., Director of R&D
High copper alloys for electrical contact assemblies are selected based on the optimum balance of strength, conductivity, and low cost. Most copper-based alloys possess only two of the three. Impurities left over from lower cost production processes tend to degrade conductivity, and intentional alloying additions that increase strength similarly degrade conductivity. A generalized example of the effect of impurities on the resistivity of pure copper can be found in Figure 1.
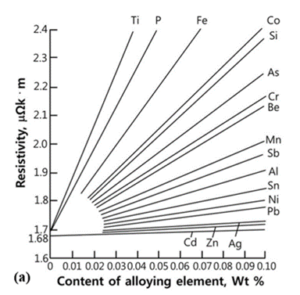
Figure 1: Effect of impurities on the conductivity of pure copper.¹
Many different relative optimums in the tradeoffs between cost, conductivity, and strength have been identified over the years resulting in a large number of copper alloys. Over time a subset of these alloys have become dominant while the others have become largely obsolete. An overview of the performance of common copper alloy grades can be found in Figure 2, with supporting data in Table 1. It should be noted that the older copper alloy grades are still referred to by the first three digits of the grade number (i.e. C10200 may also be written C102 in some cases).
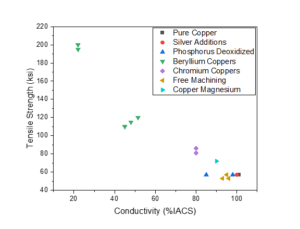
Figure 1: Effect of impurities on the conductivity of pure copper.¹
C10100 vs C10200 vs C11000
All three are grades of pure copper. C10100 is produced under the strictest controls with a nominal purity of 99.99%, C10200 is slightly lower purity at 99.95%, with C11000 being the lowest purity at 99.9%. The increased purity offers more reliable electrical performance over a wide range of service conditions. The most specific drawback is the potential of C11000 to suffer from high temperature hydrogen embrittlement due to the presence of oxygen in the copper.³
Silver Additions (C10400, C10500, C10700, C11400, C11500, C11600)
The addition of small amounts of silver results in a small increase in high temperature mechanical performance without a corresponding loss in conductivity.³
Phosphorus-Deoxidized (C12000, C12200)
Phosphorus is a cost-effective deoxidizing agent in copper reducing the risk of high temperature embrittlement, but residual phosphorus in the melt has a pronounced negative effect on conductivity.³
Free Machining Coppers (C14500, C14700, C18700)
The additions of tellurium, sulfur, and lead, respectively, create copper bars that are easier to machine compared to the more pure copper grades. This ease of machining comes with a trade off in conductivity, and, in the case of C18700, the lead additions are not Restriction of Hazardous Substances (RoHS)-compliant.³
Copper Beryllium (C17200, C17300, C17410, C7500, C17510)
The addition of beryllium adds a strong age hardening response creating a range of very high strength copper alloys. Strengths of these alloys can be 3x higher compared to other low alloy grades but the strength comes with a high conductivity debit, which can be as low as 20% of pure copper.³ These are also among the most expensive alloys discussed here.
Copper Chromium (C18150, C18200)
The addition of chromium offers a favorable pathway to moderately increase the strength without trading too much conductivity. These alloys are also age hardenable. However, maximum hardness is achieved by aging from the cold worked condition.³
Copper Magnesium (C15500)
A wrought hardenable copper alloy that retains a high level of conductivity while offering increased room temperature and elevated temperature strengths.³
Deringer-Ney is experienced in working with most common copper grades for the manufacture of electrical contact assemblies ranging from microcontact assemblies for precision measurement applications to large contacts used in power transmission and distribution infrastructure.
References:
- Han, S. Z., Choi, E. A., Lim, S. H., Kim, S., & Lee, J. (2021). Alloy design strategies to increase strength and its trade-offs together. Progress in Materials Science, 117, 100720.
- Copper.org (n.d.). Copper Alloys Advanced Search. Retrieved June 7, 2022 from https://alloys.copper.org/
- Braunovic, M “Arcing Contact Materials” Electrical Contacts: Principles and Applications, edited by Slade, Paul G. CRC press, 2017.pp. 240-243
