Issue 029, February 28, 2022
Megan K. Puglia, Ph.D., Sr. Research Chemist
Understanding specific pathways to corrosion resistance in metals and alloys is imperative to achieving high functioning, cost-effective, and stable materials. One widely-used and advantageous conduit of corrosion resistance is metal passivity. Most will agree that the passivation of a metal results from the formation of a film on the surface of the metal, which acts as a diffusion barrier. These diffusion-barrier films protect the metal surface from its environment and decrease the corrosion reaction rate of the metal surface. These films are mechanically stable and, in this context, formed spontaneously in specific environments.
Examples of these films include outer oxide layers, such as those that spontaneously form in air and aqueous environments on chromium and titanium.[1] Titanium, titanium alloys, and stainless steels are self-passivating materials, forming a stable oxide layer to some degree in air without additional chemical treatments. Self-passivating materials are beneficial because they are able to repair damage to their passivating film caused by mechanical wear.
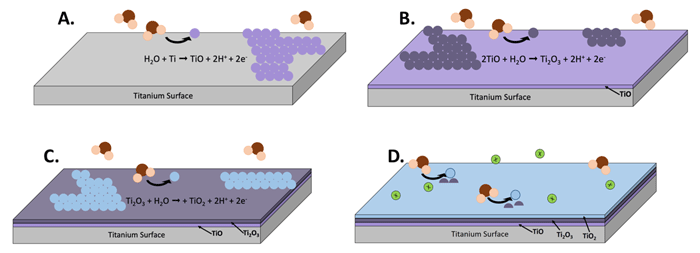
Figure 1: Schematic of the formation of a titanium oxide passivating film on titanium surface via anodic oxidation. A. Water reacts with Ti to form a TiO film. B. Water reacts with the newly formed TiO film to form Ti2O3. C. The Ti2O3 film reacts with water to form TiO2. D. The self-healing oxide films protect the buried titanium surface from corrosion reactions.
304 and 316 stainless steels (SS) are both commonly used in industrial and medical applications. Stainless steel (Fe-Cr-Ni-based alloys) passivation occurs as a result of the chromium content in the alloy. Chromium forms a strong protective oxide layer of Cr2O3 when passivated. In contrast, the oxide film that forms on mild steel or pure iron is thought to consist of one conductive inner layer of Fe3O4 connected to an outermost layer of γ-Fe2O3.[2] Fe2O3, commonly known as rust, is flaky and unstable, resulting in lower corrosion resistance in non-stainless iron alloys.
Titanium alloys also rely on passivation for their stability and corrosion resistance, especially as medical implants. The passive film on titanium alloys (TiO2) forms spontaneously in air but methods have been developed to strengthen or thicken this passivating film including glow discharge plasma deposition, hydrogen peroxide treatment, thermal oxidation, and anodic oxidation (Figure 1).These TiO2 films, which can grow from 2 nm to 20 µm depending on the formation method, act as excellent corrosion barriers against the different constituents of biological environments.[3]
Spontaneously formed diffusion-barrier layers can be enhanced by specific chemical treatments that can either increase layer thickness or change film composition. A common SS passivation enhancement process involves an acid solution (usually concentrated nitric acid, HNO3) removing Fe from the SS surface leaving behind a surface with a high percentage of Cr. The Cr then reacts with the oxygen in the air to form the stable, protective chromium oxide layer.
Passivation enhancement procedures are not necessarily ‘one size fits all’ and some changes may be made for different alloys and part geometries. Additionally, welded parts are sometimes not good candidates for passivation as weld zones often react differently to passivation procedures than the un-welded area. For example, not all chromium containing steels can be passivated, as both chromium content as well as other alloying additions like carbon and nickel can strongly affect passivation. Passivation is not usually suitable for electronic applications as passive films contribute to high contact resistance.
Deringer-Ney manufactures a number of passivated SS parts for medical applications per ASTM 967-05[4], which is the standard specification for the chemical treatment of SS parts. This specification gives instructions on cleaning, descaling, and passivating SS, as well as test methods and acceptance criteria for the treated surfaces. A number of specific tests are also outlined in ASTM 967 that can be done on passivated SS parts to determine the presence of free-iron, or other surface contaminants, that would disrupt the chromium oxide barrier-film.
Deringer-Ney Inc. is experienced in making and selling passivated stainless-steel parts—especially for medical applications—per ASTM standards, and uses this expertise to design cost-efficient, stable parts for specific applications and environments.
References:
[1] Revie, R. Winston, and Herbert Henry Uhlig. Corrosion and Corrosion Control: An Introduction to Corrosion Science and Engineering. 4th ed. Hoboken, N.J: Wiley-Interscience, 2008.
[2] Jones, Denny A. Principles and Prevention of Corrosion. 2nd ed. Upper Saddle River, NJ: Prentice Hall, 1996.
[3] Ma, Kai, Rui Zhang, Junlong Sun, and Changxia Liu. “Oxidation Mechanism of Biomedical Titanium Alloy Surface and Experiment.” International Journal of Corrosion 2020 (August 13, 2020): 1–9. https://doi.org/10.1155/2020/1678615.
[4] Standard Specification for Chemical Passivation Treatments for Stainless Steel Parts. ASTM 967-05, in Annual Book of ASTM Standards, vol. 01.03. DOI: 10.1520/A0967-05
