Issue 020, November 9, 2021
Megan K. Puglia, Ph.D., Sr. Research Chemist
Resistivity (ρ) defines a material’s ability to impede electrical current (Equation 1). Equation 1 defines resistivity mathematically where R is resistance, A is the cross-sectional area of the material, and l is the length of the material.[1] The SI unit used to express resistivity is the ohm meter (Ω∙m).
Equation 1:
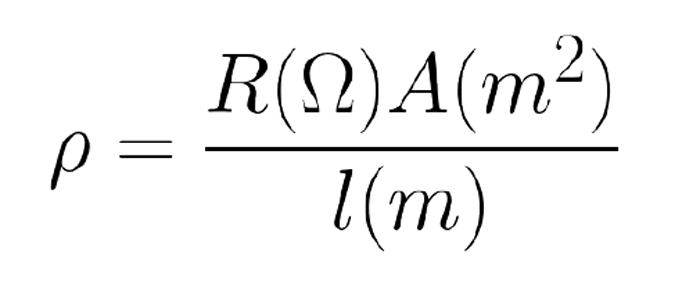
Resistivity is the inverse of conductivity (σ), which defines a material’s ability to allow electrons to flow through it per a specified unit size (Equation 2).[1] The SI unit for conductivity is siemens per meter (S/m).
Equation 2:
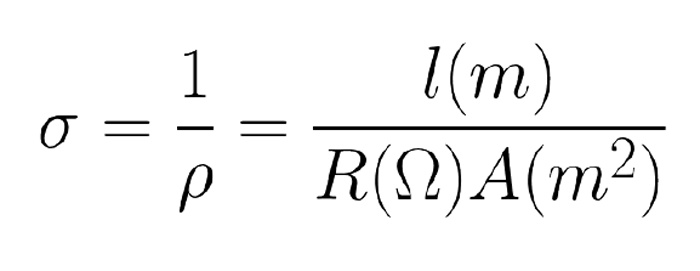
Importantly, resistivity and conductivity are properties of a material that are nominally independent of size and shape. Resistance, on the other hand, is a characteristic of a component, and can be changed by modifying part dimensions.
Resistivity in a metal is heavily influenced by the mean free path of a freely moving (valence) electron in its crystal lattice. The mean free path is the average distance an electron can travel between scattering events, or collisions, that knock the electron off its original route through the material. Scattering events increase resistivity, and are caused by irregularities in the crystal structure of metals. Examples include strain fields around solute atoms, grain and particle boundaries, and dislocations. Usually, material strengthening mechanisms increase scattering events and therefore decrease metal conductivities. Consequently, conductivities of metals that have undergone cold working, thus introducing dislocations, are lower than annealed materials of the same composition.[2] However, interestingly, resistivity of an alloy generally decreases during age hardening; solute atoms are sequestered in precipitates, rearranged by long range ordering, etc., thereby reducing their participation in electron scattering.
Resistivity and conductivity are also affected by the temperature of the material. An increase in temperature causes an increase in lattice vibrations (phonons), which cause more structural disorder and points of collision for electrons trying to move through the material. The intrinsic conductivities of the pure elements are related to many other aspects; among them are valence electrons, crystal structure, and electron density near the Fermi energy level.[2]
Resistivities of metals and other materials that are good conductors are often reported in units of microhm∙cm, whereas insulating materials have their high resistivity values reported in ohm∙cm. Some handbooks and guides rank materials by their percent conductivity relative to the international annealed copper standard (IACS), known as %IACS. The IACS is an annealed copper standard held at 20°C with an accepted conductivity value of 58×10⁶ S/m [1.72 µΩ-cm]. Therefore, a reported conductivity of 50% IACS implies that the material has half of the conductivity of an annealed copper standard, or 27.5 x10⁶ S/m [3.45 µΩ-cm].
Silver, copper, and gold are reported as having the highest conductivities of all metals due, in part, to each of their single valence electrons that is easily excited to be free-moving through their crystal lattice.[2] Note that pure silver and copper are both more likely to experience tarnish in comparison to gold and other noble metals in certain environments, thereby increasing contact resistance.[3] In addition, a higher conductivity usually indicates decreased material strength, as previously mentioned. For these reasons, it is important to choose contact materials that balance conductivity, strength, nobility, and cost appropriately for the intended application, and avoid narrow focus on bulk conductivity alone.
Deringer-Ney, Inc. makes a number of alloys with a range of combinations of resistivity, hardness, nobility, and cost to accommodate a broad spectrum of different applications and environments as seen in Table 1.[1]
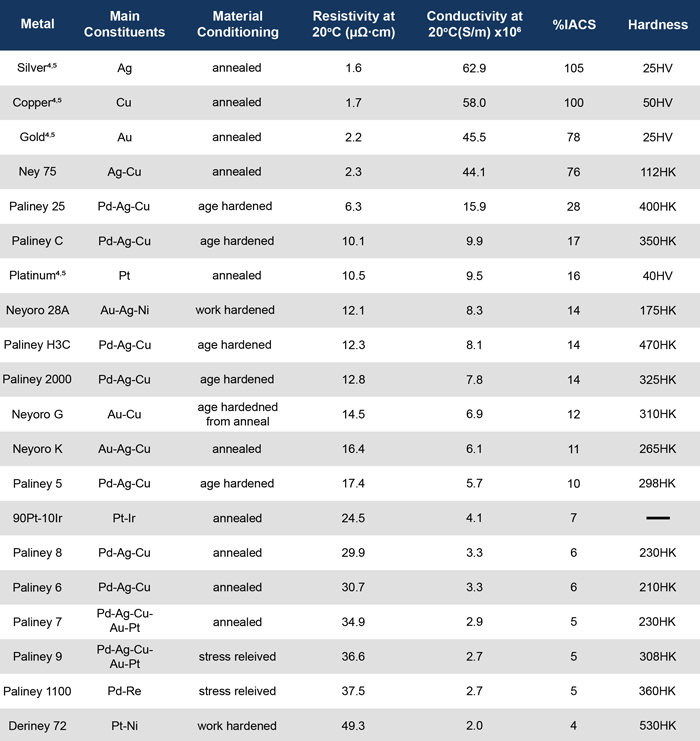
Table 1: Estimated resistivity, conductivity, and %IACS values for various metal alloys. For information on other DNI alloys or alternate tempers please visit deringerney.com. All values are estimates. Pure metal resistivity and hardness values from reference 4 and 5, respectively. Values corresponding to Knoop hardness measurements are indicated by HK, and values corresponding to Vickers hardness measurements are indicated with HV.
References:
[1] Pitney, Kenneth E. Ney Contact Manual: Electrical Contacts for Low Energy Uses. 1st ed., The J.M. Ney Company, 1973.
[2] Carter, Giles F. Principles of Physical and Chemical Metallurgy. ASM International, 1979
[3] Bowen, P. K. “Basic Theory of Contact Resistance” Deringer-Ney Tech Briefs, December 2, 2020. https://www.deringerney.com/resource-library/.
[4] Haynes, William M., et al. “Properties of Solids.” CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data: 2012-2013, CRC Press, Boca Raton, FL, 2012, pp. 12–41-12–42.
[5] Brandes, Eric A., and Colin James Smithells. Smithells Metals Reference Book. 6th ed., Butterworths, 1983.
