Issue 016, September 02, 2021
Patrick K. Bowen, Ph.D., R&D Manager
Sliding electrical contacts in aggressive environments provide a unique challenge for material selection. First, materials at the contact interface must maintain a low contact resistance and low degree of wear[1]. Applications in fuel level sensing in automobiles, for instance, currently require a lifetime greater than 6 × 106 full cycles and 107 dither cycles on the potentiometric assembly that relays fuel level to the car computer. As an analog device, the electrical contact must also maintain a high degree of signal integrity throughout a lifetime immersed in gasoline[2].
Second, the bending beam that supplies the normal force at the contact interface must resist deformation and fracture. In conductive liquid environments, modes of mechanically induced corrosion are of paramount concern. Corrosion modes of particular relevance include stress corrosion cracking (SCC) for bending beams held at constant deflection, and corrosion fatigue cracking (CFC) for beams that frequently cycle through various levels of deflection. Detailed review of these corrosion modes may be found in Jones[3].
An engineered solution available from Deringer-Ney uses a low contact resistance material based on a noble metal bonded to a high-strength flexure that does not contain costly precious metal. The design and application of the composite contact, or “wiper,” is illustrated in Figure 1.
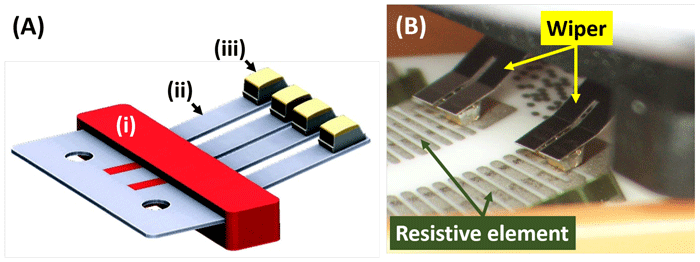
Figure 1: (A) Rendering of a composite wiper, illustrating (i) plastic over-molding, (ii) high-strength mechanical component made from base metal, and (iii) the noble metal electrical contact; and (B) Picture of the interior of a potentiometer comprising a similar composite wiper bearing on a resistive element fabricated using thick-film technology.
Contact materials have ranged from hard Pd-Ag-Cu-based materials, like Paliney® 6, to simple, soft alloys of gold. Hard electrical contact alloys are usually specified to combat abrasive wear. This may be accomplished either by matching material hardnesses on both sides of the contact pair (two-body abrasive wear), or by maximizing sliding contact hardness to reduce material removal by very hard particles (three-body abrasive wear). Softer materials, like gold, are sometimes selected to maximize material transfer from the sliding contact to the mating surface. Optimization of adhesive transfer can introduce a thick coating on the opposite surface, as shown in Figure 2. The interface effectively becomes a gold-on-gold electrical contact, with matched hardnesses and a correspondingly low rate of gold loss from the contact system.
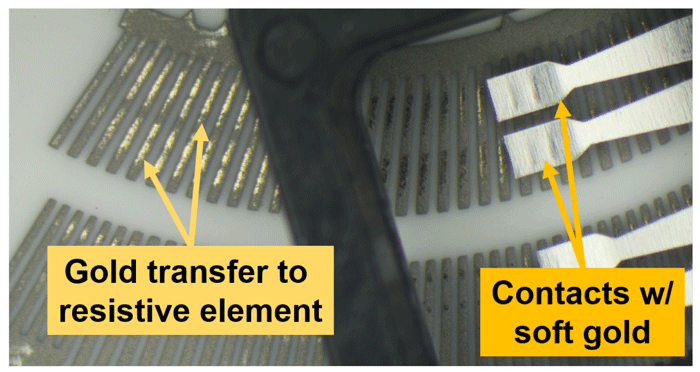
Figure 2: Example of a potentiometer showing transfer of a soft gold electrical contact to the thick-film resistive element
Flexures have been successfully constructed from alloys of copper, nickel, and even titanium. Selection of one or another alloy is usually driven by a combination of strength, fatigue, corrosion, joining, and cost considerations. Techniques have been developed that enable a considerable range of noble metal contact materials to be welded to the aforementioned base metals. In one challenging case, a soft, gold-based alloy with a low melting point has been employed as the working contact with a high strength titanium flexure.
A high degree of customizability makes composite electrical contacts with noble metals on high-strength base metal flexures a powerful design option in electronic systems.
References:
[1] Pitney KE. Ney Contact Manual: Electrical Contacts for Low Energy Uses. Bloomfield, Connecticut: The J. M. Ney Company; 1973. Available from: https://www.deringerney.com/publications/ney_electrical_contact_manual/
[2] Smith E, Ireland H. Contact systems for use in sulfur containing, reformulated gasolines. In: Electrical Contacts, 2004 Proceedings of the 50th IEEE Holm Conference on Electrical Contacts and the 22nd International Conference on Electrical Contacts. IEEE; 2004. p. 289–296.
[3] Jones DA. Principles and Prevention of Corrosion. Saddle River, NJ: Prentice Hall; 1996.
