Issue 030, March 18, 2022
Wade Jensen, Ph.D., Senior Research Metallurgis
Alloys are commonly created through melting and liquid mixing at high temperatures where most systems are completely miscible. This technique provides a relatively easy means to achieve a homogeneous mixture; however, once the homogeneous liquid solidifies, different kinds of solid mixtures may occur as the system evolves toward equilibrium.
Solid Solution – Solid solutions, or miscible mixtures, occur when one or more solute is able to substitute the base constituent on the crystal lattice, without forming a new crystal structure. There are some systems, like Ag-Au, that exhibit full miscibility. These atoms will freely occupy random lattice points on the FCC unit cell for any composition, Figure 1.a). Other systems, like Au-Fe, have limited solubility and can only incorporate solute up to the solvus curve and any additional solute will form a distinct, secondary phase. The Hume-Rothery rules postulates the conditions required to create a solid solution: 1) the atomic radii cannot differ by more than 15%, 2) similar crystal structures, 3) similar valency, and 4) similar electronegativity¹.
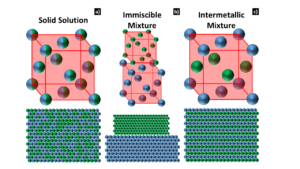
Figure 1: Diagrams depicting the crystal structures and the (111) close-packed planes for :equiatomic face centered cubic alloy exhibiting: a) a solid solution and b) an immiscible mixture; and an L10 crystal structure exhibiting c) an intermetallic mixture
Immiscible Mixture – Immiscible metal mixtures, like Ag-Cu, have limited solubility and are unable to create a homogeneous solid solution. Solute atoms are not incorporated into the crystal structure and are rejected into their own crystallographic phase. As illustrated in Figure 1.b), Ag and Cu are immiscible at high concentrations due to the difference in atomic radii rather than crystal structure. Solubility increases with higher temperatures and small quantities of solute can form a solid solution; however, a decrease in temperature will result in solute rejection.
Intermetallics Mixture – Intermetallic alloys are ordered compounds that contain two or more constituents. Specific lattice points can only be occupied by specific elements and this creates an ordered crystal structure. Intermetallic compounds are often a fixed or nearly fixed stoichiometry, and are represented by chemical formulae, for example: AuCu, β-FeSi2, or Pd2MnSb. Intermetallic alloys are generally brittle and can have high melting temperatures. The Au-Cu system has an example of an intermetallic alloy, which is engendered by an ordering process at low temperatures². Equiatomic compositions undergo an ordering from a faced-centered cubic (FCC) to an L10 crystal structure³. The constituents form alternating layers of Au and Cu along the [001] direction, as illustrated in Figure 1.c).
Deringer-Ney materials are designed by the precise alloying of solute into base precious metals. Tailoring one or more of the structural responses discussed above enables a great degree of engineering flexibility. It allows our alloy development process to select for material properties such as strength/hardness, ductility, corrosion resistance, or electrical conductivity, while often minimizing intrinsic metal cost.
References:
- W. Callister, 4.3 Impurities in Solids, in: Materials Science and Engineering and Introduction, 7th ed., John Wiley and Sons, Inc., 2007: pp. 83–85.
- R.S. Toth, H. Sato, Antiphase Domains in Ordered Au3Cu Alloys, Journal of Applied Physics. 35 (1964) 698–703. https://doi.org/10.1063/1.1713439.
- Raymond W, Cribb F, Gedeon MJ, Grensing FC. Copper-Nickel-Tin Spinodal Alloys. Adv Mater Process. 2013;20.
- Singh S, Wanderka N, Murty BS, Glatzel U, Banhart J. Decomposition in multi-component AlCoCrCuFeNi high-entropy alloy. Acta Mater. 2011 Jan;59(1):182–90.
- Bowen PK, Birdsall D, Smith EF, Laitila EA. Elastic modulus and structural changes upon age hardening of a palladium-based alloy, Paliney 7. TMS Annual Meeting & Exhibition; 2018 Mar 11; Phoenix AZ.
