Issue 001, November 18, 2020
Megan K. Puglia, Ph.D., Sr. Research Chemist
Inductively coupled plasma optical emission spectroscopy (ICP-OES) is an elemental identification and quantification technique used across many industries including geoscience, pharmaceuticals, and materials science. ICP-OES has become a common analytical technique in the precious metals industry as a complement to x-ray fluorescence spectroscopy due to its efficiency and accuracy. The composition of metal alloys, including impurities, can be accurately identified using ICP-OES either in solid form or after being digested into an aqueous solution with detection limits ranging from 0.03-1.0 ng/mL[1], although practical limits of detection in materials certification are more commonly 100-1,000 ng/mL (0.1-1 ppm).
ICP-OES works by ionizing argon gas into plasma in a radio frequency inductive torch, and introducing samples directly into the plasma. The high electron density of the plasma excites the sample, promoting electrons in it to higher energy levels. As excited electrons relax to lower energy states, they release energy in the form of photons of characteristic wavelengths. The photons are separated by wavelength, and intensities of each specific wavelength determined by a photomultiplier tube or a photodetector. Elements can produce photons of several different wavelengths yielding a specific emission spectrum with multiple characteristic peaks used to identify that element in an unknown sample.[2]
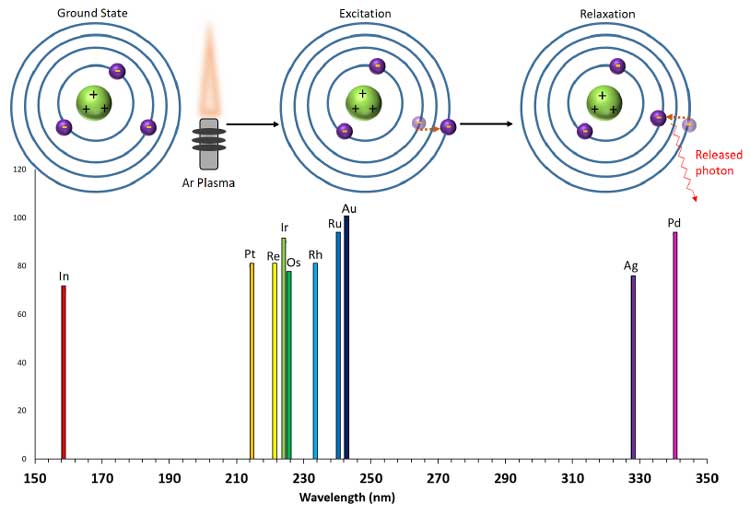
Figure 1: Scheme of ICP-OES excitation of an electron after an atom is introduced into an argon plasma
flame. The intensities of each elemental peak scale in proportion to its concentration in the unknown sample.
In the metallurgical laboratory, ICP-OES conveniently allows samples to be tested via solution or solid form. Samples in solid form are laser ablated into fine particles before being introduced into the ICP-OES system via argon gas carrier. In the case of samples that are not easily ablated due to their small size, shape, or non-homogeneity, they may be digested in acidic media and tested via solutions analysis on the same instrument.
Both solutions and solids are tested using standardized samples with known elemental composition, and instrument response characterized by standard curves over a defined range of concentrations. Unknown samples should always be run alongside standard samples with known compositions and impurity levels as a control. A specific energy is maintained in the plasma used to excite samples, and, consequently, samples with higher levels of dissolved solids are subjected to lower overall excitation than samples with fewer dissolved solids and, thus, lower matrix effects. It is therefore important to analyze standard samples with similar matrix effects to the unknown samples for accurate results.[3] Other limitations of ICP-OES include the inability to accurately detect H, O, and N, and the high limits of detection for halogens (hundreds of ppb). In addition, spectral overlap can occur between different elements which requires expertise to identify and remedy.[4]
However, ICP-OES analysis has many advantages including the simultaneous determination of about 70 elements, a wide linear range of standard curves, low limits of detection (ppb), and high sample throughput. In addition, Zr, Ta, B, and rare earth metal elements can be analyzed despite presenting challenges for atomic absorption spectroscopy.[1] These advantages in combination with its low limits of detection and speed of analysis make ICP-OES an important tool for metallurgists attempting to accurately determine the elemental compositions and impurities in metals and metal alloys.
References
[1] “Atomic Spectroscopy, A Guide to Selecting the Appropriate Technique and System.” Atomic Spectroscopy Detection Limits, PerkinElmer, Inc., 2018, www.perkinelmer.com/atomicspectroscopy.
[2] Boss, Charles B., and Kenneth J. Fredeen. “Concepts, Instrumentation and Techniques in Inductively Coupled Plasma Optical Emission Spectroscopy.” 3rd ed., PerkinElmer Instruments, 2004.
[3] “Inductively Coupled Plasma Atomic (Optical) Emission Spectroscopy (ICP-AES, ICP-OES) Fundamentals.” ICP-OES & ICP-AES Spectrometers, SPECTRO Analytical Instruments GmbH (Ametek Materials Analysis Division), 2020, www.spectro.com/products/icp-oes-aes-spectrometers.
[4] Olesik, John W. “ICP-OES Capabilities, Developments, Limitations, and Any Potential Challengers?” Spectroscopy, vol. 35, no.6, 1 June 2020, pp. 18-21
