Issue 036, May 23, 2022
Megan K. Puglia, Ph.D., Sr. Research Chemist
Noble metals are crystalline solids at room temperature, meaning that their atoms are ordered along a three-dimensional repeating pattern called a lattice. The hard sphere model describes the behavior of this crystallographic lattice¹; 1) atoms are spheres that can be in contact with each other, but they can never interpenetrate, and 2) atoms tend to optimize their arrangement to maximize stability and packing density (also known as close-packing). The most common structure for pure metals and substitutions alloys are also the most close-packed: body-centered cubic (BCC), hexagonal closed-packing (HCP), and face-centered cubic (FCC). Noble metals², as shown in Figure 1, are only found in FCC and HCP structures, but this is not to say that intermetallic alloys preclude alternate crystal structures.
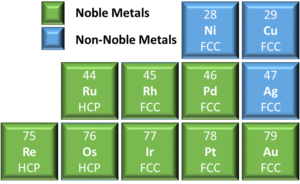
Figure 1: Truncated periodic table of elements depicting the crystal structure for noble and non-noble metals as defined by Deringer-Ney².
Metallurgists represent the infinitely repeating lattice with a more manageable “unit cell”, which is the simplest repeating unit needed to recreate the crystal. The unit cell provides copious information about stoichiometry, isotropy, and characterization³. Ductility and the accommodation of plastic deformation can also be explained with crystallography.
Certain orientations of the unit cell contain close packed planes and close packed directions, which together are also known as slip systems. There is no spacing between atoms in these slip systems, and atoms can easily slide into a neighboring position. This facilitates a facile mode for dislocation movement and the number of available slip systems directly correlates to ductility.
Body Centered Cubic: BCC crystals have cubic unit cells with an atom at the center and, as shown in Figure 2, there are close packed directions but no true close packed planes. However, taking into account the most densely packed plane, there can be up to 48 slip systems. BCC metals often experience a ductile to brittle transformation at cryogenic temperatures; this is due to these slip systems requiring heat to activate dislocation movement. This barrier for dislocations migration makes BCC metals less ductile than FCC metals, but still more ductile that HCP metals.
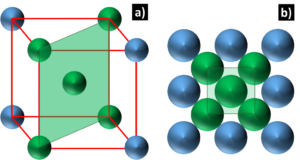
Figure 2: Diagram depicting a) the BCC unit cell and b) the most densely packed plane, denoted by the green plane and the green atoms that lie on this plane.
Hexagonal Close-Packed: There are a total of 14 different lattice systems and crystals are not always arranged along cubic lattices³. The HCP structure is one such crystal and it has a hexagonal unit cell, Figure 3. There is only one close pack plane per unit cell and only three slip systems. This is not enough to facilitate plastic deformation and HCP crystal structures like Re, Ru, and Os are known for their poor accommodation of plastic deformation. Some HCP crystals, like Ti, are able to take advantage of additional densely packed planes that improve their ductility.
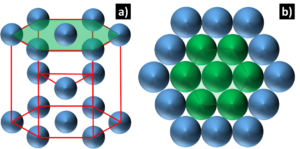
Figure 3: Diagram depicting a) the HCP unit cell and b) the close packed plane, denoted by the green plane and the green atoms that lie on this plane.
Face Centered Cubic: FCC crystals also have cubic unit cells, but with an atom on each cube face. As shown in Figure 4, all the atoms on this plane are touching their nearest neighbors. This configuration has both close pack planes and directions and engenders a total of 12 slip systems. This allows for facile dislocation movement and makes FCC crystal structures such Pd, Pt, Ag, Au, and their substitutional alloys highly ductile. Dislocation movement here is not temperature dependent and is not impeded by cryogenic temperatures. DNI alloys are based on the FCC crystal structure, and so they possess high ductility and wide range of service temperatures in addition to nobility from their Pd, Pt, or Au base composition.
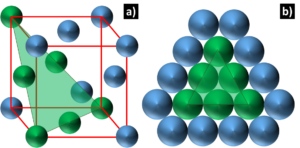
Figure 4: Diagram depicting a) the FCC unit cell and b) the close packed plane, denoted by the green plane
and the green atoms that lie on this plane.
References:
- W. Callister, 3 The Structure of Crystalline Solids, in: Materials Science and Engineering and Introduction, 7th ed., John Wiley and Sons, Inc., 2007: pp. 38–73.
- M. Puglia, What Determines Deringer-Ney Alloy Nobility?, (2020) 1–2.
- Kittel, Charles, 1 Crystal Structure, in: Introduction to Solid State Physics, 8th ed., John Wiley & Sons, 2005: pp. 1–22.
